【Poster_MBSJ 2018】Improvement of the translation efficiency of proline residue-containing proteins using the reconstituted cell-free protein synthesis sytem (PUREfrex)
Download PDF (1 MB)
DownloadsKeywords
PUREfrex® 2.0 / EF-P / proteins containing consecutive prolines / Release Factor 2 (RF2) / AmiB / PfkB / IL-6 / Pro-GFP / DHFR-Pro
Abstract
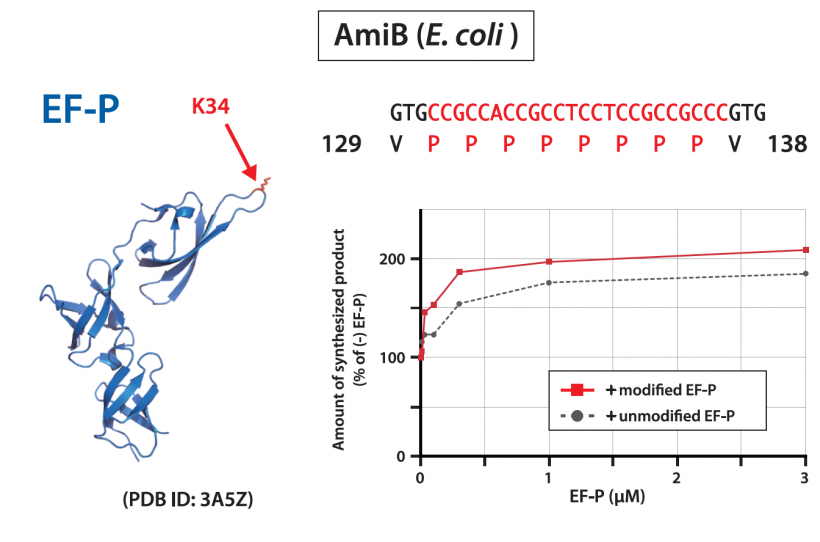
PUREfrex® is a build up cell-free protein synthesis system (PURE system) that reconstitutes the factors involved in transcription and translation in E. coli. With the improvement of the reaction mixture, the synthesis amount has been increased up to about 1 mg/ml, and it is now possible to synthesize disulfide bond-containing proteins such as antibodies (IgG) in active form. However, it is also observed that the amount of synthesis is low due to the sequence of the protein. In such cases, one solution is to replace the codon with a synonymous codon to increase the amount of protein synthesis. On the other hand, if the cause is the amino acid sequence, the sequence cannot be changed, so the reaction mixture must be adjusted. Here, we show an example of improving the synthesis of a protein whose amount of synthesis is reduced due to proline by adding a factor to the reaction mixture.
In the synthesis of proteins containing consecutive prolines (such as Pro-Pro-Pro), a translation factor called EF-P is involved in E. coli, but PUREfrex® does not contain EF-P. We tested the effect of EF-P by synthesizing continuous proline-containing proteins in the reaction mixture of PUREfrex® supplemented with EF-P. As a result, it was confirmed that the amount of synthesis was increased by about twofold with the addition of EF-P. When proteins containing proline at the N-terminus are synthesized in PUREfrex®, it is observed a decrease in the amount of synthesis. The mature form of IL-6 contains proline near the N-terminus, and when synthesized in PUREfrex®, a byproduct with a molecular weight lower than the proper molecular weight was observed. Therefore, we tested the effect of EF-P. As a result, the byproducts almost disappeared and the amount of the target product synthesized increased. When a protein with a C-terminal proline residue was synthesized in PUREfrex®, the translation efficiency was low when the termination codon was UGA, but the use of a release factor (RF2) with higher activity increased the amount of protein synthesis.
These results indicate that the use of PUREfrex® with the addition of EF-P or translation factors with high specific activity can improve the translation efficiency of proteins containing proline residues.